In electrolysis an electrode connected to the chargers positive terminal is called anode. This is represented by the.

Electrolysis Process On Passing Electric Current The Cations Move Towards The Cathode And Get Deposited Piscinas De Agua Salada Escuela De Natacion Piscinas
The anode is also called sacrificial or waste electrode because it can be expected to disintegrate.

. The Anode is the negative or reducing electrode that releases electrons to the external circuit and oxidizes during and electrochemical reaction. Oxidation-reduction reaction is a process in which the degree. CuSO 4 aq Cu 2 aq SO 4 2 H 2 O l H aq OH aq During electrolysis.
Copper sulphate and water ionise as. 4H 4e- 2H 2. The cathode was derived from the Greek word- kathodos.
During the electrolysis of aq. The charge of the anode and the cathode depends on whether it is a Galvanic cell spontaneous chemistry driving electricity or an electrolysis cell non-spontaneous chemistry driven by. Anode and Cathode in Electrolysis By fu_Hayley478 02 Sep.
2H 2 O O 2 4H 4e-Cathode Reaction. Since the electrons were discovered a cathode is defined as negative and an anode is defined as positive. Alkaline electrolyzers operate via transport of hydroxide ions OH- through the.
2Br- Br2 2e- A silvery grey liquid will form at the cathode molten lead though this is difficult to see as it will sink to the bottom as it is denser than the electrolyte. The anode of the galvanic cell gives off electrons and return from the circuit into the cell through the cathode. 55 74 votes.
Electrolysis is the process which occurs in an electrochemical cell when electrons pass from the anode to the cathode via an external circuit connecting the electrodes. Electrodes are plates of certain materials connected to each other which pass electricity through themselves anode and cathode. How and why the mass of anode and cathode change during electrolysis.
H ions are attracted to the cathode gain electrons and form hydrogen gas OH- ions are attracted to the anode lose electrons and form oxygen gas The overall balanced equation for. The anode is usually the positive side. Electrolysis Of Copper Sulfate Solution With Impure Copper Anode And Pure Copper Cathode Copper.
Please explain it thoroughly. The anode is the electrode where electricity moves into. Water is split at the cathode in order to form H 2 and release hydroxide anions OH that cross through the diaphragm and combine to form O 2 at the anode.
Inside the battery the anode is where the negative ions lose electrons and these electrons move from the anode to the cathode which is electron rich. The Cathode is the. K2SO4 using pt-anode and pt-cathode hydrogen gas liberated at cathode and oxygen gas at anode.
Electrolysis of aqueous copper sulphate solution. An electrolytic cell occurs when a positive potential voltage is applied to one electrode anode and a negative potential voltage to the other electrode cathode. Electrolysis anode and cathode.
Electroplating Metal Cathode Electrolysis When metal ions are reduced they. Therefore energy is applied. The cathode is the electrode where electricity is given out or flows out.
State what is formed at the cathode and at the anode during the electrolysis of the following subjects. How do you find anode and cathode. Solution The anode is a reducing agent because its behaviour will reduce ions at.

Electrolysis Of Copper Sulfate Solution With Impure Copper Anode And Pure Copper Cathode Copper Purification Technology Educat Pure Products Copper Solutions

Electrolysis Chemical Changes Electrochemistry Energy

Look4chemistry Electrolytic Cell Electrochemistry Cell Positive And Negative
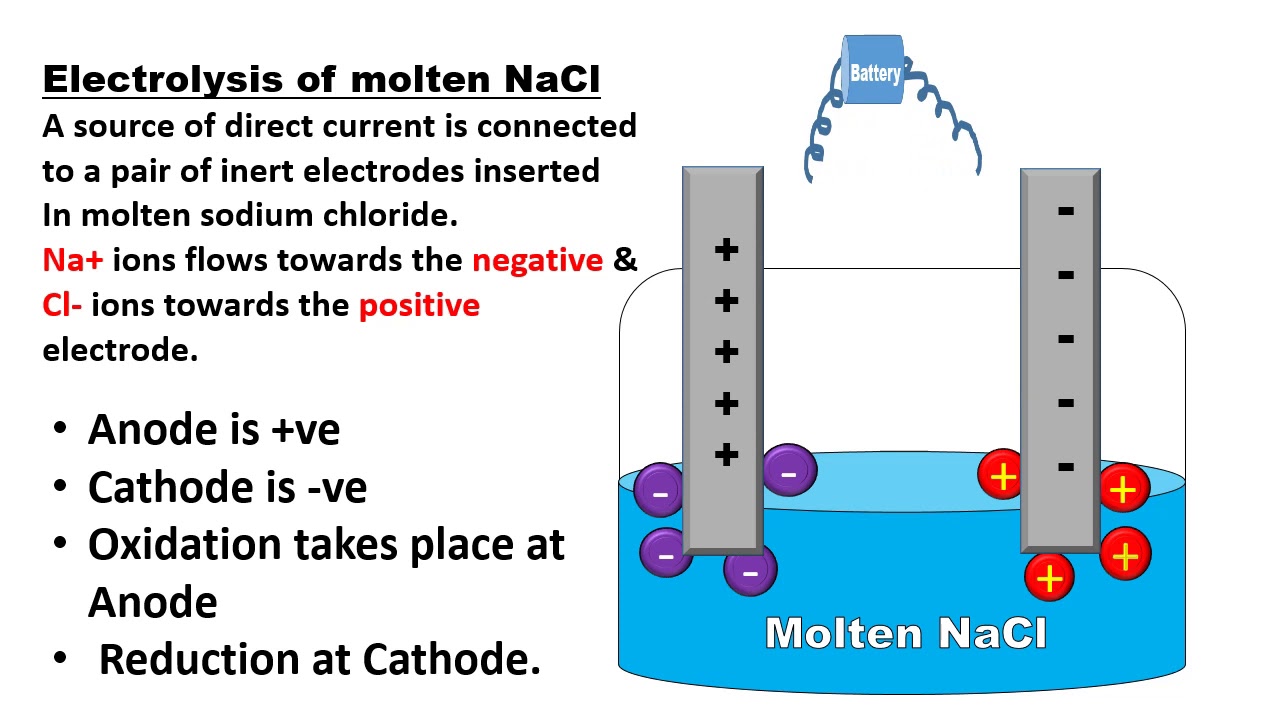
What Is Electrolytic Cell Electrochemistry Chemistry Basics Chemistry
0 Comments